Average Evaporation Rate Of Boiling Water . Web although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. This equation helps researchers and engineers calculate. Explain the relationship between vapor pressure of water and the capacity of air to hold. Web you need to know the rate of heat given by the flame to the water. By the end of this section, you will be able to: Suppose the flame transfers $h$ kj/s to the water. Web decreased the atmospheric pressure results in decreased partial pressure of water, hence a lower humidity. Web decreased the atmospheric pressure results in decreased partial pressure of water, hence a lower humidity. Web the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more things than you. Web the absolute temperature of the boiling water, in kelvins (k). At a pressure greater than 1 atm, water.
from mungfali.com
Suppose the flame transfers $h$ kj/s to the water. Web decreased the atmospheric pressure results in decreased partial pressure of water, hence a lower humidity. Web the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more things than you. Explain the relationship between vapor pressure of water and the capacity of air to hold. Web decreased the atmospheric pressure results in decreased partial pressure of water, hence a lower humidity. Web although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. By the end of this section, you will be able to: Web you need to know the rate of heat given by the flame to the water. At a pressure greater than 1 atm, water. This equation helps researchers and engineers calculate.
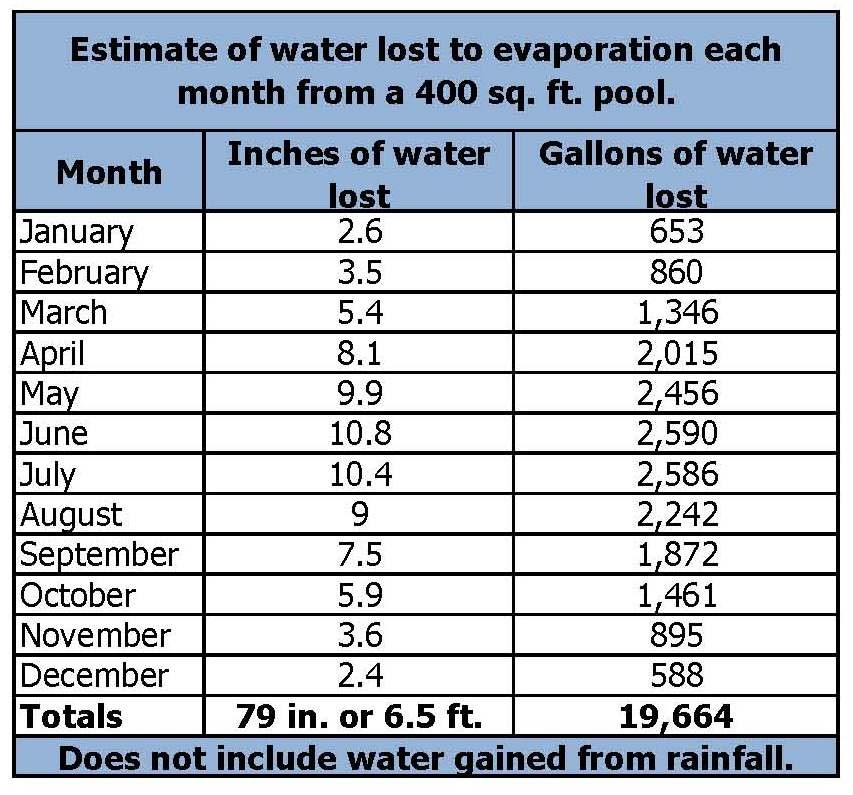
Pool Evaporation Chart
Average Evaporation Rate Of Boiling Water Web the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more things than you. This equation helps researchers and engineers calculate. At a pressure greater than 1 atm, water. Explain the relationship between vapor pressure of water and the capacity of air to hold. Suppose the flame transfers $h$ kj/s to the water. Web decreased the atmospheric pressure results in decreased partial pressure of water, hence a lower humidity. Web the absolute temperature of the boiling water, in kelvins (k). Web the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more things than you. Web you need to know the rate of heat given by the flame to the water. Web although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. Web decreased the atmospheric pressure results in decreased partial pressure of water, hence a lower humidity. By the end of this section, you will be able to:
From 88guru.com
Difference Between Evaporation and Boiling Average Evaporation Rate Of Boiling Water Web the absolute temperature of the boiling water, in kelvins (k). At a pressure greater than 1 atm, water. Web decreased the atmospheric pressure results in decreased partial pressure of water, hence a lower humidity. This equation helps researchers and engineers calculate. Web the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on. Average Evaporation Rate Of Boiling Water.
From thewaterfiltermarket.com
How Long Does It Take for Water To Evaporate? Water Filter Market Average Evaporation Rate Of Boiling Water By the end of this section, you will be able to: Web decreased the atmospheric pressure results in decreased partial pressure of water, hence a lower humidity. Suppose the flame transfers $h$ kj/s to the water. This equation helps researchers and engineers calculate. Web the answer is yes, the rate that water evaporates can indeed be calculated, but it depends. Average Evaporation Rate Of Boiling Water.
From saylordotorg.github.io
Vapor Pressure Average Evaporation Rate Of Boiling Water Explain the relationship between vapor pressure of water and the capacity of air to hold. At a pressure greater than 1 atm, water. Web although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. By the end of this section, you will be able to: Web the answer is yes, the. Average Evaporation Rate Of Boiling Water.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Average Evaporation Rate Of Boiling Water Web decreased the atmospheric pressure results in decreased partial pressure of water, hence a lower humidity. Web you need to know the rate of heat given by the flame to the water. Web the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more things than you. Suppose the flame transfers. Average Evaporation Rate Of Boiling Water.
From 88guru.com
Vaporization Types, Examples and Factors affecting the Rate of Average Evaporation Rate Of Boiling Water Web you need to know the rate of heat given by the flame to the water. At a pressure greater than 1 atm, water. Explain the relationship between vapor pressure of water and the capacity of air to hold. Suppose the flame transfers $h$ kj/s to the water. Web the answer is yes, the rate that water evaporates can indeed. Average Evaporation Rate Of Boiling Water.
From www.researchgate.net
The vapor pressure of ethanol vs. the normal boilingpoint temperature Average Evaporation Rate Of Boiling Water By the end of this section, you will be able to: At a pressure greater than 1 atm, water. This equation helps researchers and engineers calculate. Web decreased the atmospheric pressure results in decreased partial pressure of water, hence a lower humidity. Web the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on. Average Evaporation Rate Of Boiling Water.
From www.teachoo.com
What is the Difference between Evaporation and Boiling? Class 9 Average Evaporation Rate Of Boiling Water Web although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. Web the absolute temperature of the boiling water, in kelvins (k). Web decreased the atmospheric pressure results in decreased partial pressure of water, hence a lower humidity. Explain the relationship between vapor pressure of water and the capacity of air. Average Evaporation Rate Of Boiling Water.
From www.researchgate.net
Evaporation rate of a water NaCl solution versus the environment Average Evaporation Rate Of Boiling Water Web although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. Web the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more things than you. Web decreased the atmospheric pressure results in decreased partial pressure of water, hence a lower humidity.. Average Evaporation Rate Of Boiling Water.
From morioh.com
Vapor Pressure Normal Boiling Point & Clausius Clapeyron Equation Average Evaporation Rate Of Boiling Water Web the absolute temperature of the boiling water, in kelvins (k). At a pressure greater than 1 atm, water. Explain the relationship between vapor pressure of water and the capacity of air to hold. Suppose the flame transfers $h$ kj/s to the water. Web decreased the atmospheric pressure results in decreased partial pressure of water, hence a lower humidity. Web. Average Evaporation Rate Of Boiling Water.
From chart-studio.plotly.com
Evaporation Rates scatter chart made by Guimonda1 plotly Average Evaporation Rate Of Boiling Water Web you need to know the rate of heat given by the flame to the water. Web although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. This equation helps researchers and engineers calculate. Suppose the flame transfers $h$ kj/s to the water. Web decreased the atmospheric pressure results in decreased. Average Evaporation Rate Of Boiling Water.
From www.researchgate.net
Comparison between daily‐average evaporation rates calculated from in Average Evaporation Rate Of Boiling Water Web decreased the atmospheric pressure results in decreased partial pressure of water, hence a lower humidity. Web the absolute temperature of the boiling water, in kelvins (k). This equation helps researchers and engineers calculate. Web the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more things than you. By the. Average Evaporation Rate Of Boiling Water.
From mungfali.com
Pool Evaporation Chart Average Evaporation Rate Of Boiling Water Web you need to know the rate of heat given by the flame to the water. Web although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. Explain the relationship between vapor pressure of water and the capacity of air to hold. Suppose the flame transfers $h$ kj/s to the water.. Average Evaporation Rate Of Boiling Water.
From www.researchgate.net
Effect of evaporation rate using Activated carbon and methanol Average Evaporation Rate Of Boiling Water Web the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more things than you. This equation helps researchers and engineers calculate. Web you need to know the rate of heat given by the flame to the water. At a pressure greater than 1 atm, water. Web although we usually cite. Average Evaporation Rate Of Boiling Water.
From ppt-online.org
theory of ideal gases презентация онлайн Average Evaporation Rate Of Boiling Water Explain the relationship between vapor pressure of water and the capacity of air to hold. Web you need to know the rate of heat given by the flame to the water. This equation helps researchers and engineers calculate. Web although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. Suppose the. Average Evaporation Rate Of Boiling Water.
From www.semanticscholar.org
Figure 1 from Problems with determination of evaporation rate and Average Evaporation Rate Of Boiling Water Web the answer is yes, the rate that water evaporates can indeed be calculated, but it depends on a few more things than you. Web although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. Suppose the flame transfers $h$ kj/s to the water. Web the absolute temperature of the boiling. Average Evaporation Rate Of Boiling Water.
From schematicmaxeywheezle.z21.web.core.windows.net
Normal Boiling Point On Phase Diagram Average Evaporation Rate Of Boiling Water By the end of this section, you will be able to: At a pressure greater than 1 atm, water. Web although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. Web the absolute temperature of the boiling water, in kelvins (k). Web you need to know the rate of heat given. Average Evaporation Rate Of Boiling Water.
From www.pnas.org
Evaporation rate of water in hydrophobic confinement PNAS Average Evaporation Rate Of Boiling Water Explain the relationship between vapor pressure of water and the capacity of air to hold. By the end of this section, you will be able to: At a pressure greater than 1 atm, water. Suppose the flame transfers $h$ kj/s to the water. Web decreased the atmospheric pressure results in decreased partial pressure of water, hence a lower humidity. Web. Average Evaporation Rate Of Boiling Water.
From chem.libretexts.org
10.4 Properties of Liquids Chemistry LibreTexts Average Evaporation Rate Of Boiling Water Web the absolute temperature of the boiling water, in kelvins (k). Suppose the flame transfers $h$ kj/s to the water. Web decreased the atmospheric pressure results in decreased partial pressure of water, hence a lower humidity. At a pressure greater than 1 atm, water. Web you need to know the rate of heat given by the flame to the water.. Average Evaporation Rate Of Boiling Water.